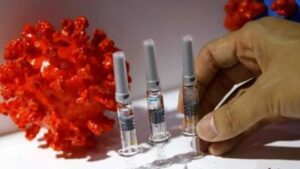
Photo Credits: REUTERS
India has given an exception to the foreign manufactured vaccines to fight Covid-19 from conducing local bridging studies prior to going for emergency use authorization, on the condition that they have already got an approval from any one of the major regulators globally, as declared by the government on Tuesday.
The move is an attempt made by the Centre to fasten the procedure of emergency use authorization for foreign manufactured Covid-19 vaccines which have been granted approval. The Union Health Ministry said in a statement, “The National Expert Group on Vaccine Administration for Covid-19 (NEGVAC), after comprehensive deliberation, recommended that vaccines for Covid-19, which have been developed & are being manufactured in foreign countries and which have been granted emergency approval for restricted use by USFDA, EMA, UK MHRA, PMDA Japan or which are listed in WHO(Emergency Use Listing) may be granted emergency use approval in India, mandating the requirement of post-approval parallel bridging clinical trial in place of conduct of local clinical trial as per the provisions prescribed under Second Schedule of the New Drugs & Clinical Trials Rules 2019.”
The expert vaccine panel recommended that the first 100 beneficiaries of these foreign vaccines shall be reviewed for seven days for safety outcomes prior to further vaccination across the country.
The ministry statement further added, “The Union government, after due consideration, has accepted the recommendation of NEGVAC. This decision will facilitate quicker access to such foreign vaccines by India & would encourage imports including import of bulk drug material, optimal utilisation of domestic fill and finish capacity etc., which will in turn provide a fillip to vaccine manufacturing capacity and total vaccine availability for domestic [use].”
This subject was further discussed in he 23rd meeting of NGEVAC scheduled on April 11, which was headed by Dr VK Paul, member (health) of NITI Aayog.
Melukote - Vairamudi Festival 2022. Latest Photos and Videos of Lord Cheluvanarayanaswamy. Video Footage of… Read More
The Bengaluru Metro Rail Corporation (BMRCL) revealed that Namma Metro will be functional from 7… Read More
India has appealed the EU member states to distinctly consider extending exemption for those who… Read More
Prime Minister Narendra Modi will address several beneficiaries of the Digital India initiative on the… Read More
Private hospitals in the country will no longer be able to obtain the Covid-19 vaccines… Read More
The Directorate General of Civil Aviation (DGCA) took the call for further extending the ban… Read More
This website uses cookies.
Leave a Comment