Bharat Biotech, though filed for emergency use authorization for its vaccine candidate Covaxin, Vydehi hospital, based in Bengaluru is still battling to find adequate volunteers who can participate in the clinical trials.
The trials via the hospital commenced on December 2, the hospital had a plan to administer the primary dose of vaccine to a count of 1,000 volunteers in a week. But, only 400 volunteers have registered by December 8 for participation in the clinical trial.
The volunteers have to undergo prior screening for assessing their system sustainability for the trial. The hospital is also catering to the travel arrangements for larger groups of people which includes resident’s welfare associations and apartments – who wish to be vaccinated under the clinical trials move. The hospital has also provided an alternative of reimbursement pertaining to travel cost.
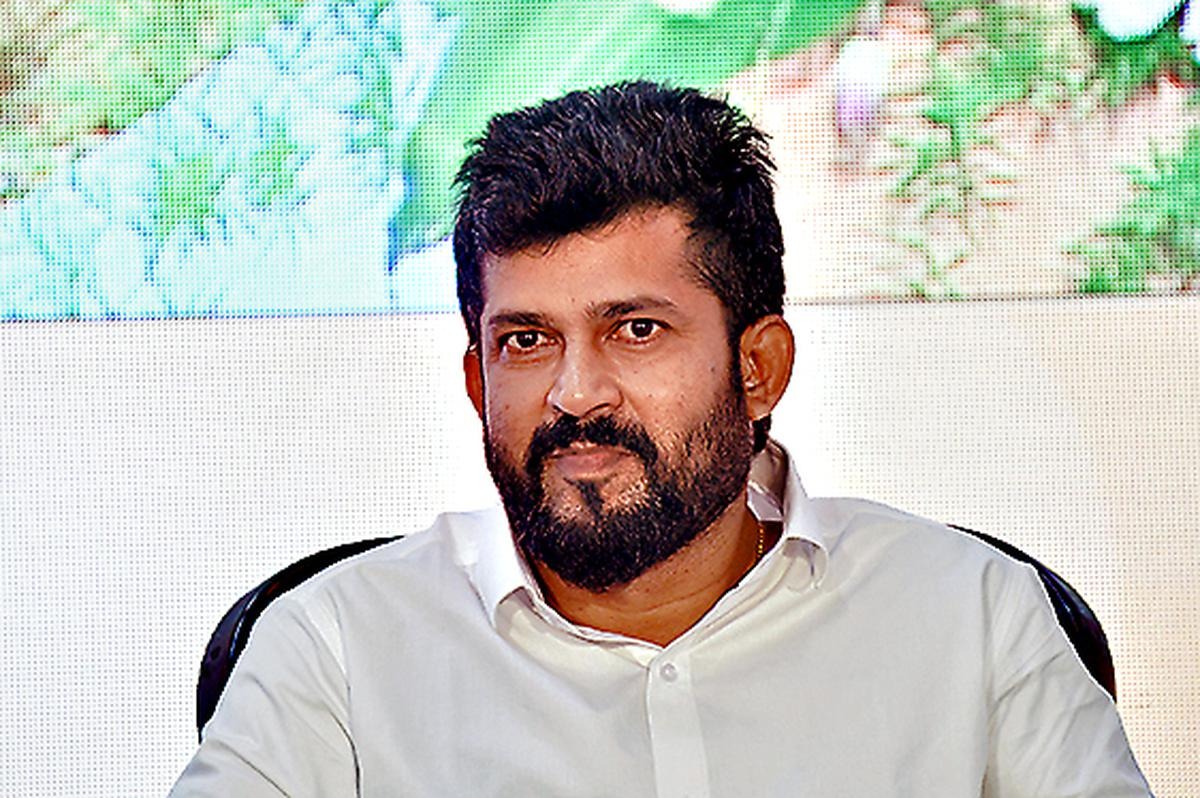
Dr Rajesh Naidu, practising orthopaedic at Vydehi hospital and Director of Clintrac International Private Limited, an independent organization conducting the clinical trials of the vaccine stated they were being positive towards attaining the target.
Dr Naidu said, “We were trying to reach a target of 1,000 volunteers in one week. We are asking everyone. People have to come forward. Hopefully by the end of next week, we hope to inoculate 1,000 volunteers. Other centers started early and so there is more awarenessss. We only started recently. It will pick up slowly.”
The hospital iterated about giving first shots as volunteers enrol, which will go on till Bharat Biotech registers 26,000 volunteers from the 25 trial sites across the country. He further added, “The screening of the volunteers will take at least a day. If interested volunteers are coming from far in groups of 10 or 20, we can provide transport. If people have apprehension of coming to a hospital, we have a separate entrance, passage and wing for them.”
The vaccine trials for Covaxin will be two-dose based schedule, given at a gap of 28 days. The efficacy of the vaccine candidate will be assessed after 14 days of administering the second dose. The undergoing trials for Covaxin will be the largest clinical trials in India.
Dr Naidu stated, “Once the ICMR has the data for the booster dose of 26,000 volunteers, we will stop giving the first shot to more people. Till this number is reached, we will vaccinate as and when the volunteers come forward without a cut-off date.”
The clinical trial team members from Clintrac for Covaxin have asked anyone interest to get in touch with the coordinator – Nripesh Nepal at 9739419272 or give a call to Vydehi hospital by dialling 1692 as extension number and ask for connecting with Covaxin clinical trial room.